Mahnke Auto Body’s Aluminum Advantage
Mahnke Auto Body understands the extreme responsibility the company commits itself to with the challenges and dangers of repairing
aluminum. Mahnke Auto Body invested in Ford and Honda OEM aluminum
certifications, expensive high-end aluminum repair equipment,
intensive employee training, and the creation of protected workspaces
in order to repair aluminum vehicles.
Dangers of Alumninum
How is aluminum dangerous? Aluminum dust is flammable. NASA used
aluminum in solid fuel rocket boosters.
“Solid rocket fuel is the original rocket fuel, dating back to the early fireworks developed by the Chinese centuries ago. For the SLS boosters, aluminum powder serves as the fuel and
Mahnke Auto Body has instituted proper safety protocols, including protected workspaces for repairing aluminum to prevent explosions, mixed metal contamination, galvanic corrosion, and health hazards. Below are a few key factors when working with aluminum:
- Aluminum dust is flammable.
- Steel and Stainless Steel Corrodes Aluminum.
- Aluminum c
orrodes galvanized steel. - Health hazards from breathing aluminum dust.
- Aluminum dust in an auto body shop must be and mixing aluminum dust.
Todd Hoffman, vice president of Hoffman Ford in Harrisburg, Pa. “From what we understand from the big players in the paint business and body repair, it is a pretty significant safety risk to mix steel and aluminum.”
“If it happens, it’s ugly,” I-CAR industry technical support manager Steve Marks said referencing an aluminum explosion.
Marks, who has done training for aluminum adopters Land Rover and Jaguar, also noted that steel and aluminum can mix to create thermite for a “really nasty” explosion.
You want to make sure equipment is grounded to avoid static sparks, he said, and you’ll want to use brushless methods of removing the aluminum to avoid sparks. Aluminum dust is also very fine and “does float a lot longer,” according to Marks. That raises issues both with flammability and with potential health risks from breathing it. And technicians trying to remove aluminum dust should not use compressed air, he said.”
The 2015 Ford F-150: The Pioneer of Modern Aluminum in Automobiles
OEM certifications give Mahnke access to up-to-the-minute information about the proper operating procedures for repairing the multitude of materials used light-weighting vehicles. “The 2015 F-150” chart shows all the elements in the construction of this truck.
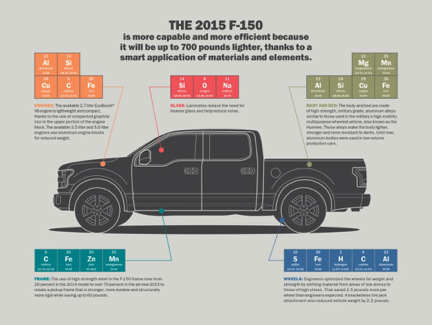
Above image courtesy
“Great care must be taken, not only with aluminum but knowing how to treat the trim on painted auto-body steel, dissimilar metal fasteners, electroplated bumpers, galvanized steel, and dissimilar cooling system components. The resulting problems and mitigative measures for controlling galvanic corrosion are presented.”
Science question: How can you tell the difference between aluminum and magnesium? Test by pouring vinegar on the piece. Magnesium will react and the vinegar will fizz, also the metal will discolor at the point at contact. Nothing will happen with aluminum.
A Brief History of Aluminum
Aluminum, in its metal form, is not found naturally, even though aluminum is the third most common element in the Earth’s crust. Humans have used aluminum compounds and salts for thousands of years in making fine pottery, mordants for dyes, and medical purposes, including astringents.
Aluminum reacts with water and oxygen to form powdery oxides and hydroxides, which is why the metal is never found in nature. Extracting aluminum is very expensive and energy-intensive, even today.

Aluminum: An Ancient Metal
First century Roman savant,
Renaissance Alchemists and Chemists of the Enlightenment believed that substances which did not decompose were elements, therefore intuiting an element to be associated with the alum’s white crystals. However, identifying the true nature of aluminum remained elusive until 1808, when English Chemist Humphry Davey determined the base of alum was metallic.
At first, Davey named the element “Aluminum,” then renamed it “Aluminium” to be in line with the nomenclature of the expanding list of newly discovered elements. Davey is also credited for the discovery and naming of; potassium, sodium, magnesium, calcium, strontium, and barium. Americans and Canadians spell and pronounce the name “aluminum,” while the British (and most of the rest of the world) use the spelling and pronunciation of aluminum.
Davey discovered aluminum could be produced by electrolytic reduction from alumina (aluminum oxide

Image above courtesy of: https://www.miningreece.com/portfolio/bauxite/
Until the revolutionizing discovery of aluminum ore in 1821, materials from which to extract aluminum were scarce. French geologist Pierre Berthier was first to discover the rich source of alumina, from which aluminum can be extracted, which he named “bauxite” after the local village, Les Baux, Provence, France.[ From bauxite, aluminum oxide is extracted by electrolysis of alumina, producing pure aluminum. Bauxite presently supplies 99% of the world’s aluminum and is still the primary and least expensive source.
“In 1825, Hans Christian Oersted, a Danish chemist, was the first to produce tiny amounts of aluminum. Two years later, Friedrich Wöhler, a German chemist, developed a different way to obtain aluminum. By 1845, he was able to produce samples large enough to determine some of aluminum’s basic properties.

Wöhler’s method was improved in 1854 by Henri Étienne Sainte-Claire Deville, a French chemist. Deville’s process allowed for the commercial production of aluminum. As a result, the price of aluminum dropped from around $1,200 per kilogram ($543 per lb.) in 1852 to around $40 per kilogram ($18 per lb.) in 1859.”
Two important developments in the 1880s greatly increased the availability of aluminum. The first was the invention of a new process for obtaining aluminum from aluminum oxide. Charles Martin Hall, an American chemist, and Paul L. T. Héroult, a French chemist, each invented this process independently in 1886. The second was the invention of a new process that could cheaply obtain aluminum oxide from bauxite. Bauxite is an ore that contains a large amount of aluminum hydroxide (Al2O3·3H2O), along with other compounds. Karl Joseph Bayer, an Austrian chemist, developed this process in 1888. The Hall-Héroult and Bayer processes are still used today to produce nearly all of the world’s aluminum.
With an easy way to extract aluminum from aluminum oxide and an easy way to extract large amounts of aluminum oxide from bauxite, the era of inexpensive aluminum had begun. In 1888, Hall formed the Pittsburgh Reduction Company, which is now known as the Aluminum Company of America, or Alcoa.
When it opened, his company could produce about 25 kilograms of aluminum a day. By 1909, his company was producing about 41,000 kilograms of aluminum a day. As a result of this huge increase of supply, the price of aluminum fell rapidly to about $0.60 per kilogram (.27 cents per lb.)”
Early Aluminum Cars
In 1879, German mechanical engineer Karl

Making Automobiles Lighter: Lightweighting
From the beginning, automobile designers have actively sought strong, light weight, malleable construction materials. “Lightweighting,” is to find and use attainable, practical, affordable, and sustainable ways to create lighter weight components. Lighter weight is essential to better braking, handling, fuel conservation, and the maximization of engine power.
As the price of aluminum dropped and the supply grew in the 1880s, automobile designers creatively incorporated its use into body and engine components.
“The first sports car featuring an aluminum body was presented at the Berlin International Motor Show in1899; the first internal combustion engine with aluminum parts was made two years later when in 1901 Karl Benz, of Benz and Company, presented a new car for the prestigious race in Nice, France.”
Source: https://www.anodizing.org/page/1stALCar/The-First-Aluminum-Automobiles.html
The lightweighting provided by aluminum added to the successful performance of the car, but difficulties in metal working, lack of knowledge and the high price of aluminum prohibited its use in early mass production.”
Now as then, car buyers wonder how this amazingly light metal, so easy to crumble and tear, could ever be strong enough to take a hit in a collision. The answer: Alloys, plus heat or cold treating. Aluminum, when alloyed with a variety of elements, including iron, copper, magnesium, manganese, silicon, tin and zinc and treated with heat or cold, rivals steel in strength.

“Lightweighting, driven by aluminum’s extraordinarily high strength-to-weight ratio, continues to be an important requirement in the automotive industry today. The 5000 series alloys are popular candidates to fill the rapidly growing need for automotive structural sheet. In these applications, strength and corrosion resistance are needed but formability is also an important requirement. Today, 6000 series extrusions are in use as part of automotive and light weight truck framework.”
Source: https://www.anodizing.org/page/1stALCar/The-First-Aluminum-Automobiles.html
The most widely used alloy is 7075. It consists of aluminum, zinc, magnesium, and copper. It’s the strongest of all aluminum alloys and comparable in that respect with steel, however, it weighs only a third of what steel weighs.
In 2007, Corporate Average Fuel Economy (CAFE) standards regulations went into effect and seventeen automakers, domestic and foreign, agreed together to increase the mpg and lower co2 emissions of their fleet of vehicles over a period of time. Lightweight materials include carbon fiber, magnesium, plastics, adhesives, and aluminum. All of these materials can be found in late model vehicles. Aluminum is the least expensive and most recyclable.
Aluminum’s Carbon Footprint

Image courtesy of: https://www.millercoorsblog.com/wp-content/uploads/2017/03/COorsCans1970s.jpg
Coloradans can feel a sense of pride that Coors Brewing company invented the first aluminum can, used for beer, in 1959, concurrently creating a system for recycling. Aluminum is 100% recyclable and 75% of all aluminum ever produced, is still in the system. Production of aluminum from recycled metal saves more than 90 percent of the energy that would otherwise be required by primary production.




